The Phytocannabinoid group of compounds in Cannabis.
The reason to group phytocannabinoids
Despite most people assigning the psychoactive capabilities of weed solely to ∆9-THC, various researchers have noted how the high from cannabis extracts is a combination of the cannabinoids, terpenoids, flavonoids and other compounds. It has been stated that effects from cannabis extracts are “two to four times greater than that expected from their THC content” (alone) (Carlini, Karniol, Renault, & Schuster, 1974).
There are so many compounds in cannabis, with some researchers listing over 420 compounds (Turner, Ellsohly, & Boeren, 1980) and others, another 200 compounds in the smoke alone (Sparacino, Charles, Hyldburg, & Hughes, 1990) and hence it is very helpful to group the compounds together. One group of compounds found in cannabis that will be touched on today is the cannabinoid group.
Knowing the compounds, their structure (for polarity and what solvent to use) and their boiling points (what temperature to boil off the solvent without also boiling off the compounds) can help anyone who is interested in extracting cannabinoids or just interested for interest’s sake!
Cannabinoids, sometimes called phytocannabinoids (to differentiate between synthetic cannabinoids and natural ones). Please be very aware of the dangers of synthetic cannabinoids! Phytocannabinoids consist of the terpenophenolic compounds with a 21 Carbon chain (C21) (McPartland & Russo, 2012). The main phytocannabinoids with their typical concentration, boiling points and properties are presented in Table 1 below.
Table 1: Main phytocannabinoids present in cannabis (based off McPartland & Russo, 2012).
| Structure | Concentration (% dry weight) | Boiling point (°C) | Properties |
| ∆-9-Tetrahydrocannabinol (∆-9-THC) [Figure 1] | 0.1 – 25 | 157 | Euphoriant, Psychoactive, Anti-inflammatory, Analgesic, Anti-emetic |
| Cannabidiol (CBD) [Figure 2] | 0.1 – 2.9 | 160 – 180 | Analgesic, Non-psychoactive, Anti-inflammatory, Analgesic, Anti-emetic, Antispasmodic |
| Cannabinol (CBN) [Figure 3] | 0 – 1.6 | 185 | Sedative, Antibiotic, (CBN is a byproduct of oxidation) |
| Cannabichromene (CBC) [Figure 4] | 0 – 0.65 | 220 | Anti-inflammatory, Anti-fungal, Antibiotic |
| Cannabigerol (CBG) [Figure 5] | 0 – 1.2 | 52 (melting point) | Anti-inflammatory, Anti-fungal, Antibiotic |
| ∆-8-Tetrahydrocannabinol (∆-8-THC) [Figure 6] | 0 – 0.1 | 175 – 178 | Resembles ∆-9-THC (no shit), Less psychoactive, More stable, Antiemetic |
| Tetrahydrocannabivarin (THCV) [Figure 7] | 0 – 1.4 | < 220 (less than 220) | Analgesic, Euphoriant |
- The boiling points are those measure at atmospheric pressure
Figures 1-7: Phytocannabinoids Structures
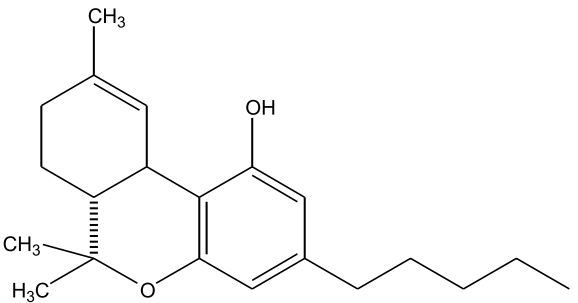
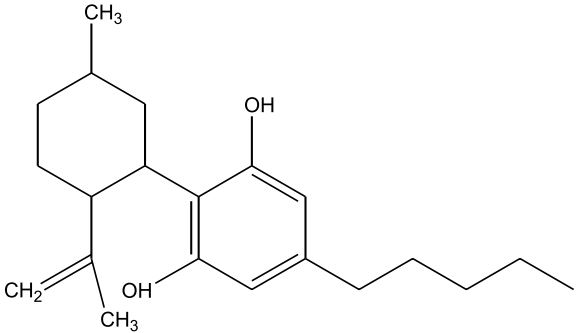
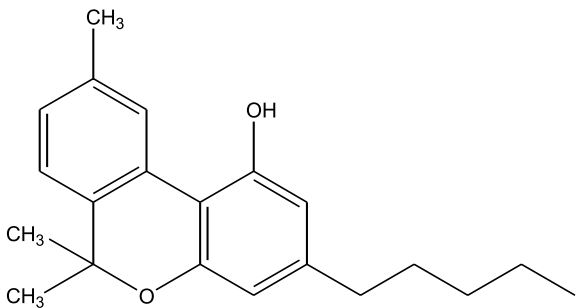
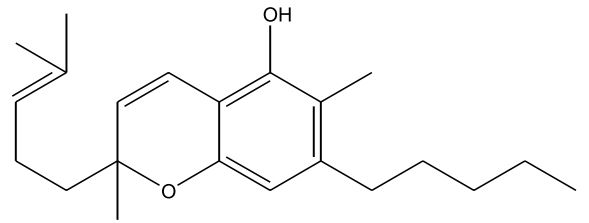
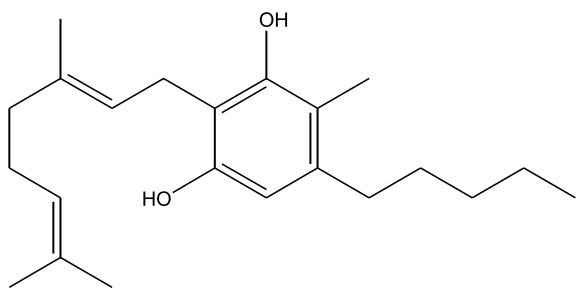
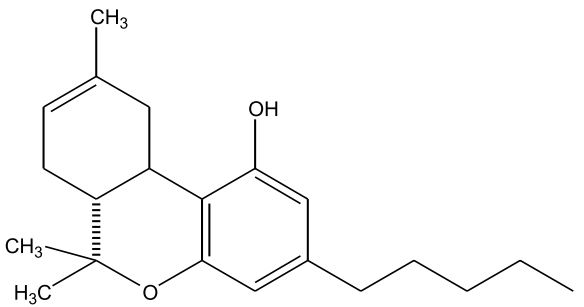
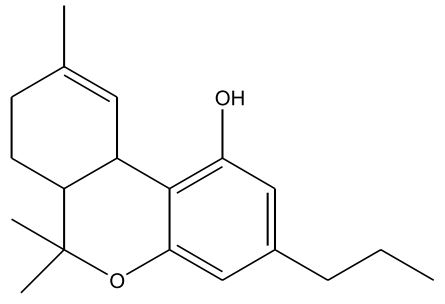
Fun fact about ingesting cannabis
The reason why one feels so much higher from edibles compared to smoking is because you are. When THC is taken orally, it metabolizes in the small intestine into 11-hydroxy THC, a metabolite that is more psychoactive than actual ∆-9 THC (Browne & Weissman, 1981). Clearly scientists even back in the day have stated that “smoking cannabis is a satisfactory expedient in combating fatigue, headache and exhaustion, whereas the oral ingestion of cannabis results chiefly in a narcotic effect which may cause serious alarm” (McPartland & Russo, 2012).
References
Browne, R. G., & Weissman, A. (1981). Discriminative Stimulus Properties of Δ 9 -Tetrahydrocannabinol: Mechanistic Studies. The Journal of Clinical Pharmacology, 21(S1), 227S-234S. https://doi.org/10.1002/j.1552-4604.1981.tb02599.x
Carlini, E. A., Karniol, I. G., Renault, P. F., & Schuster, C. R. (1974). Effects of Marihuana in Laboratory Animals and in Man. British Journal of Pharmacology, 50(2), 299–309. https://doi.org/10.1111/j.1476-5381.1974.tb08576.x
McPartland, J. M., & Russo, E. B. (2012). Cannabis and Cannabis extracts: Greater than the sum of their parts? Cannabis Therapeutics in HIV/AIDS, 1(3), 103–132. https://doi.org/10.1300/J175v01n03_08
Sparacino, Charles, M., Hyldburg, P. A., & Hughes, T. J. (1990). Chemical and Biological Analysis of Marijuana Smoke Condensate. In C. C. Nora & R. L. Hawks (Eds.), Research Findings on Smoking of Abused Substances (pp. 121–140). Rockville, Maryland: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES.
Turner, C. E., Ellsohly, M. A., & Boeren, E. G. (1980). Constituents of Cannabis Sativa L. XVII A review of the natural constituents. Journal of Natural Products.


Leave a Reply